Water H₂O Molar Mass, the most critical substance on Earth, is represented chemically as H₂O. Its simplicity belies its importance, gambling a key role in almost all natural and chemical approaches. To recognize how water interacts with one-of-a-kind substances, one essential concept to understand is its molar mass.
What is Molar Mass?
Molar mass is the mass of one mole of a given substance. In easier phrases, it presents the load of 6.022 × 10²³ particles (Avogadro’s range) of a selected detail or compound. For water, the molar mass tells us the mass of one mole of H₂O molecules.
Composition of H₂O
The chemical system H₂O well-known that each molecule of water incorporates hydrogen (H) atoms and one oxygen (O) atom. To calculate the molar mass of water, we want to determine the character loads of these atoms.
Atomic Masses of Hydrogen and Oxygen
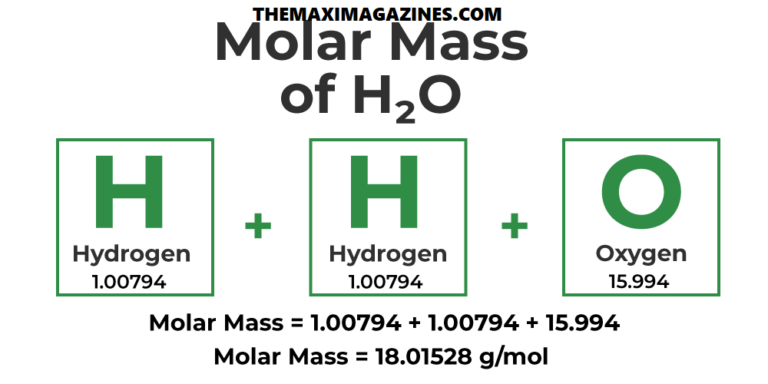
Hydrogen has an atomic mass of approximately 1.008 atomic mass gadgets (amu). Since water has hydrogen atoms, we multiply this by way of 2, which gives us 2.016 amu for hydrogen. Oxygen, however, has a higher atomic mass of round 16.00 amu.
Calculating the Molar Mass of Water
Now, to discover the total molar mass of water, we in reality add the loads of hydrogen and oxygen. This is calculated as: Molar mass of H₂O=(2×1.008)+16.00=18.016g/mol. Thus, the molar mass of water is set 18.016 grams in keeping with mole.
Why is Molar Mass Important?
Molar mass is essential in stoichiometry, the part of chemistry that offers with the quantitative relationships among reactants and products in a chemical reaction. Knowing the molar mass lets in chemists to calculate how lots of each substance is needed or produced in a reaction.
Applications in Real-Life Chemistry
In regular chemical approaches, which encompass dilution or mixing, molar mass plays a crucial function. For instance, in business chemistry, knowing the molar mass of water is essential while calculating the portions of reactants or merchandise at some point of the producing of numerous compounds.
Role in Solutions
Water, regularly known as the “everyday solvent,” dissolves many materials. Understanding its molar mass is particularly beneficial in making answers. When a solute is dissolved in water, chemists can use the molar mass to because it should be degree how a bargain solute is wanted for a desired attention.
Relation to Molecular Weight
Although molar mass and molecular weight are regularly used interchangeably, they’ve moderate variations. Molar mass is expressed in grams regular with mole, even as molecular weight is a dimensionless quantity. However, for sensible capabilities, both provide precious information about the size and mass of molecules.
Importance in Biology
Water’s molar mass also holds significance in biological structures. In cells, as an instance, the motion of water and unique molecules across membranes relies on the proper stability of materials. The functionality to degree water’s molar mass aids in understanding these crucial biological techniques.
Conclusion
In summary, the molar mass of water is a easy but essential concept in chemistry. By calculating the mass of its additives, hydrogen and oxygen, we arrive at a rate of about 18.016 g/mol. This number one understanding opens doors to severa packages, from lab experiments to commercial manufacturing and even organic functions.